Recently, the German Federal Ministry of Health has published a draft law on the financial stabilization of the statutory health insurance (GKV-FinStG). In the following figure, we have summarized the newly proposed negotiation terms. The draft law intends for a stricter regulation of price negotiations following benefit assessments depending on the status of the most economic appropriate comparative therapy (ACT). A distinction is made between:
- Generic ACT: No change to the current regulations. The new active substance is allowed to exceed the costs of the ACT if an added benefit of any extend is determined.
- Patent-protected ACT: Only new active substances with a considerable or major added benefit are allowed to exceed the costs of the ACT. The rationale behind this is that the patent-protected ACT has already undergone price negotiations with the statutory health insurers and thus the price level has already been negotiated previously. In case an ACT is patent-protected, but has not been subjected to benefit assessment according to AMNOG and therefore has not been part of any price negotiations, a discount of 15 % is applied to the annual therapy costs.
In addition to the stricter regulations for price negotiations, a 20 % discount for new active substances used in combination therapy, the introduction of a solidary contribution for the years 2023 and 2024, and the reduction of the orphan drug threshold from 50 Mio € to 20 Mio € is proposed by the new draft law. >>>click for more information>>>
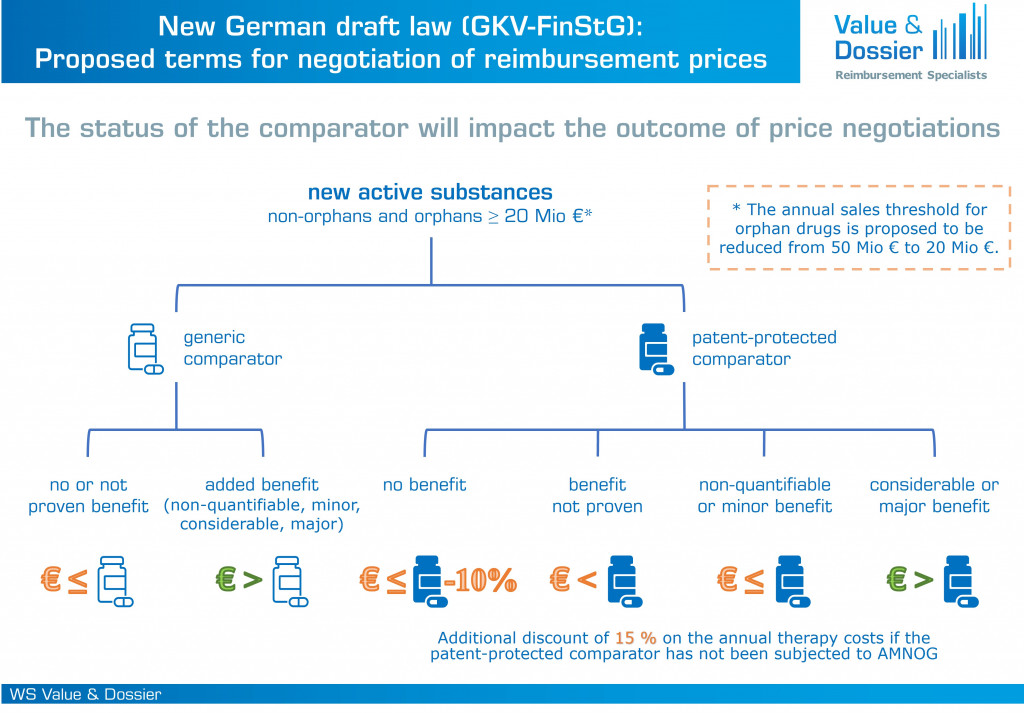
Proposed regulations of price negotiations based on the status of the most economic comparator and the extend of the added benefit according to the new German draft law (GKV-FinStG).