As part of the dialogue with the pharmaceutical industry, the German government has promised to introduce the option of a confidential reimbursement amount. The aim is to support pharmaceutical companies whose products are subject to a considerable price decrease after undergoing an AMNOG benefit assessment by offering the option to keep the new price confidential. Nevertheless, according to the newly published draft bill on Medical Research (Medizinforschungsgesetz, MFG), confidential reimbursement amounts will have the opposite effect. For the target group of products with low priced appropriate comparative therapies (ACTs), this will lead to a drastic decrease in revenue especially (Figure 1). We have taken a closer look at the draft bill and want to make you aware of the price it comes with!
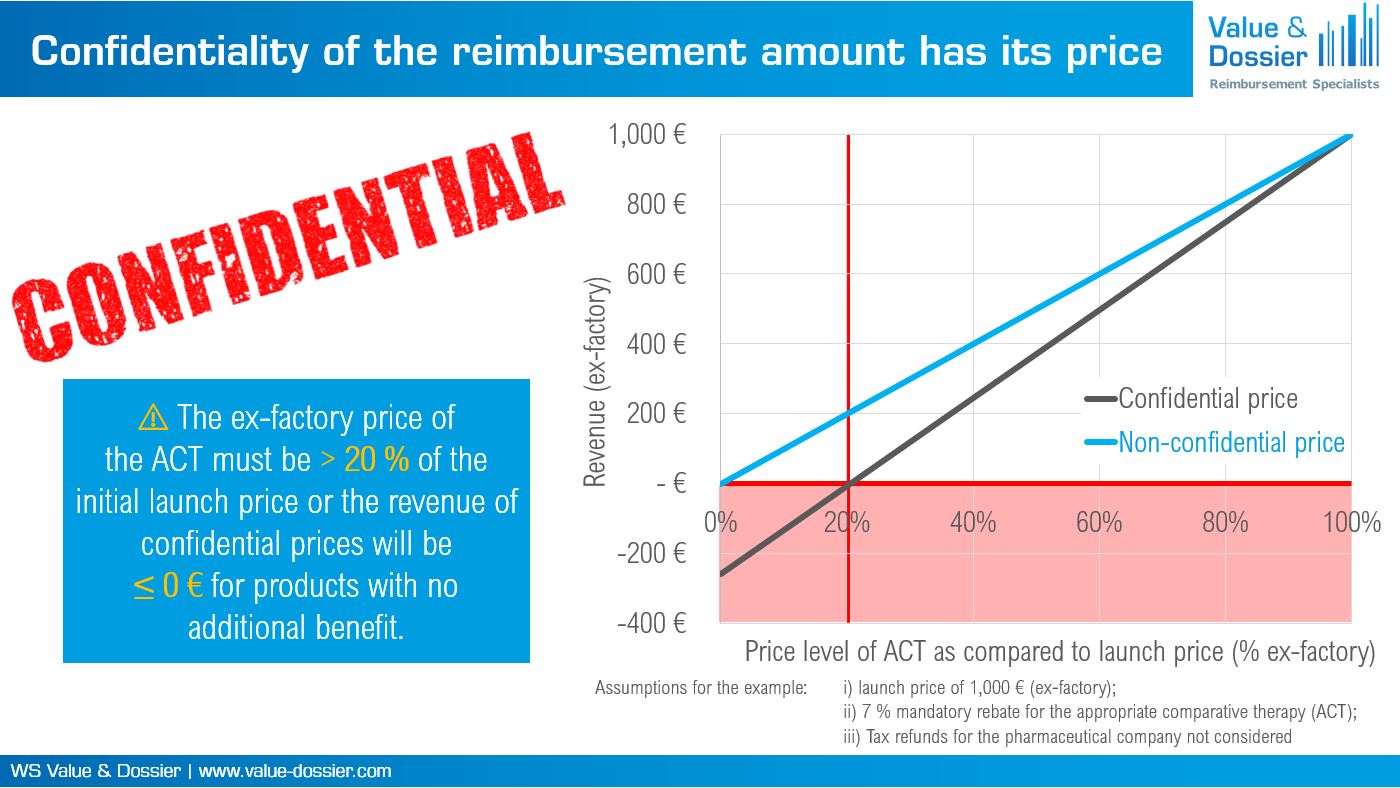
Figure 1: Consequences of confidential reimbursement amounts for the pharmaceutical company according to the new draft bill on Medical Research (Medizinforschungsgesetz, MFG).
Briefly, according to the proposed system, in future pharmaceutical companies may agree with the payer organization to keep the reimbursement amount confidential. Thus the real price would not be published in the official price lists any longer. Moreover, the difference between the list price and the real price will have to be paid back to the health insurances.
After careful reflection, it is critical to point out that this confidentiality clause comes with a price! Since the backpayment by the company shall not only include the price difference but also the wholesaler and pharmacy margin and value added tax related to the original launch price.
For instance, if a product does not have an additional benefit in comparison to the ACT, the new regulation constitutes a break-even-point at which the revenues of the pharmaceutical company will be zero. The ex-factory price of the ACT must be > 20 % of the initial launch price or the revenue of confidential prices will be ≤ 0 € for products with no additional benefit (Figure 1). Not shown in Figure 1 is that application of an additional 20 % combination rebate may be possible. Further, if the comparative therapy is still patent-protected even the same systematic applies for products with a non-quantifiable or minor additional benefit. An additional 15 % rebate applies if the patent-protected ACT was not subjected to AMNOG.
With the draft bill, due to the option of confidential reimbursement amounts European reference prices will not be used as factor in the price negotiations any longer. In respect to the additional benefit, this may be an advantage for products with an at least considerable benefit. Considering products with a lower benefit rating, the option of confidential reimbursement does not have an impact on these prices .
Overall, regarding the introduction of confidential reimbursement prices in Germany the draft bill may likely worsen the situation for products with a low benefit rating. In terms of European market access, a European reference price system would be just and more appropriate than confidentiality compared to a price penalty.