In their 2nd and 3rd reading, the German Parliament has decided further specifications of the new law on the financial stabilisation of the statutory health insurances (GKV-FinStG).
As already outlined in the draft of the law, the outcome of the AMNOG benefit assessment will further gain importance for the subsequent price negotiations. In case the appropriate comparative therapy (ACT) is patent-protected, a premium price will only be possible for products with a considerable or major added benefit. If the patent-protected ACT has not been subjected to AMNOG benefit assessment itself, further rebates will be applied. For ACTs that are not patent-protected, there are no changes, and products with an added benefit of any extent can achieve premium prices. Furthermore, only products with considerable or major added benefit will be exempted from the mandatory 20 % discount if they are used in combination with other drugs.
Currently, the added benefit of orphan drugs is proven by their market authorization and submission of a full AMNOG dossier is not necessary as long as the annual sales do not exceed 50M €. The initial draft of the GKV-FinStG law proposed a reduction of this threshold to 20M €. However, this threshold has now been increased to 30M €. The German Parliament estimates that approximately 10 orphan drug products will need to undergo reassessment after implementation of the law.
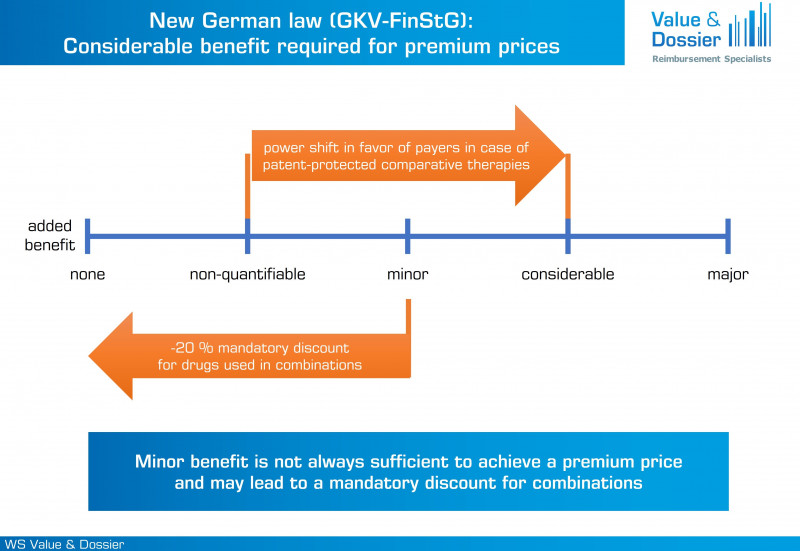
The outcome of the AMNOG benefit assessment will gain importance for the subsequent price negotiations.