WS Value & Dossier GmbH
Your specialists for market access and reimbursement
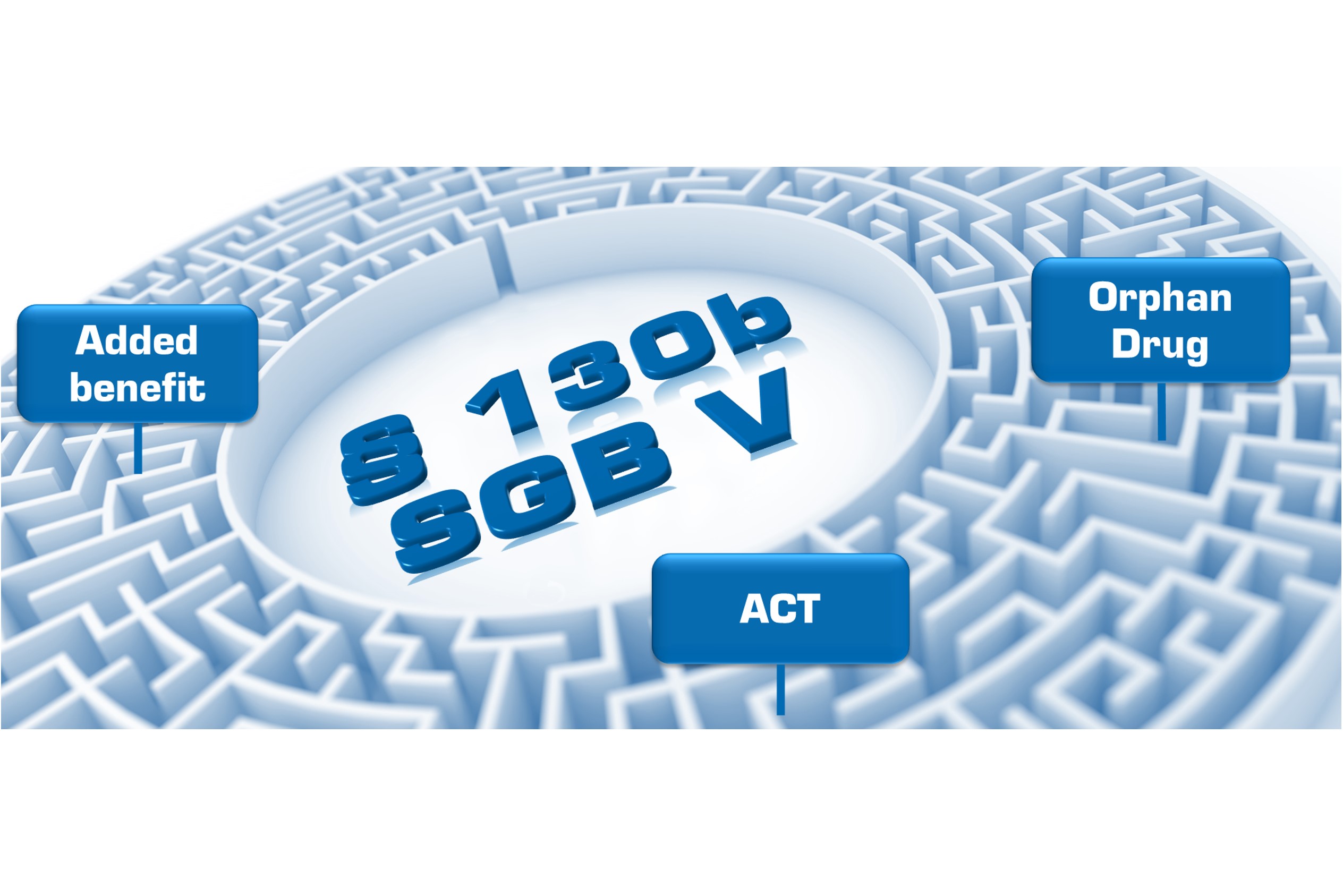
How the German AMNOG process works
Value & Dossier GmbH advises pharmaceutical companies and biotechnology companies throughout the AMNOG process and on all matters relating to market access and reimbursement. Based on our long-standing expertise, we advise you on the development of the best strategy, the preparation of your benefit dossier for the G-BA assessment and the price negotiations with the National Association of Statutory Health Insurance Funds (GKV-Spitzenverband).
Your success is what drives us. We will make sure that your new product will be reimbursed in the best possible way.
Our Services
“We offer individual and company-specific solutions focussing on the value of the product while implementing it accordingly in the required benefit dossiers.”
Recent Articles
Our new AMNOG Negotiation Guide is online now!
With the SHI Financial Stabilisation Act (GKV-FinStG), the framework conditions for price negotiations for medicinal products in
Non-randomised studies and non-adjusted indirect comparisons
Overview of our services in the field of non-randomised studies and indirect comparisons Strategic consulting Systematic research
Introduction of confidential reimbursement prices sets the wrong incentives!
We are pleased to inform you that our analysis of confidential reimbursement prices in Germany has been
 Strategic Market Access Consulting
Strategic Market Access Consulting German Benefit Assessment (AMNOG)
German Benefit Assessment (AMNOG) AMNOG price negotiations
AMNOG price negotiations  Reference pricing for pharmaceuticals
Reference pricing for pharmaceuticals European Health Technology Assessment (EU-HTA)
European Health Technology Assessment (EU-HTA) European Reference Price Calculator
European Reference Price Calculator Reimbursement of Medical Devices
Reimbursement of Medical Devices HTA Services
HTA Services