Strategic Market Access Consulting
With a population of more than 82 million, Germany is the largest market in the European Union and has a central role in European pricing, as it serves as the reference price country for the introduction of new medicines. One particular feature of Germany is that there is still free pricing at the time of market launch. The reimbursement amount is only negotiated and set after the benefit assessment in the subsequent price negotiations 12 months after market launch. As a result, Germany effectively acts as a price anchor for other European countries. The benefit assessment and the reimbursement amount are therefore of great strategic importance for the whole of Europe.
We support pharmaceutical companies with our know-how and expertise in all phases of market access and reimbursement of medicinal products in order to develop the best possible market and pricing strategy. We advise you on the specifics of the AMNOG (Act on the Reform of the Market for Medicinal Products) process, prepare the benefit dossier for you and we prepare you in the best possible way for the discussions and negotiations with the payers and the Federal Joint Committee.
Market and Price Analyses
Market and Price Analyses
Market and price analyses are a necessary prerequisite for classifying the market potential and comparable medicinal products in a given indication. We offer you indication- and company-specific analyses as a basis for the positioning of your product and preparing for later price negotiations within the AMNOG process. Our European Reference Price Calculator is a unique tool that allows you to simulate European prices in an interdependent reference price system. Our reference price calculator is available for the calculation of reference prices.
Pricing and Price Negotiation
Pricing and price negotiation of innovative medicinal products is one of the most important aspects of market access and reimbursement. We support you in pricing through market and price studies, budget impact analyses and epidemiological analyses all the way to the implementation of price studies. We offer specific training to help you optimise your preparation for negotiations on reimbursement amounts and drug prices.
Adjustment for inflation
Pursuant to § 130a (3a) Sentence 2 Social Code Book V, pharmaceutical companies may claim inflation compensation of the dispensing prices for products which are subject to the price moratorium. The base price is the price level as of August 1st, 2009, or the price of the market launch for products introduced to the market after August 1st, 2010. The inflation adjustment may be applied starting on July 1st, 2018, and the amount may be increased annually after July 1st of the subsequent years. The extent of the inflation adjustment is determined by the consumer price index of the Federal Statistical Office (Figure 1). We support you with the review and calculation for your products.
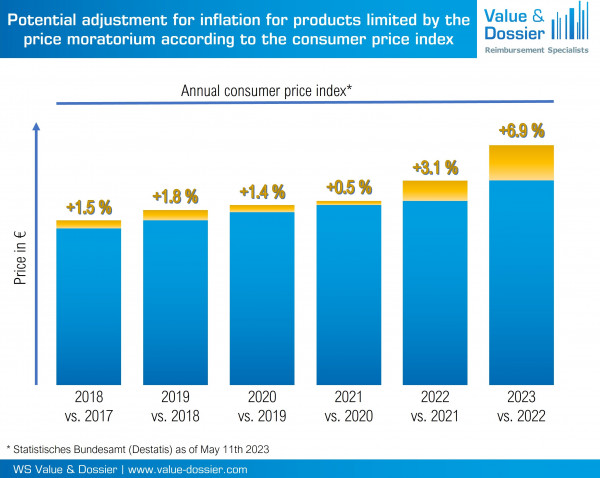
Figure 1: Adjustment for inflation according to the consumer price index of the Federal Statistical Office (Statistisches Bundesamt)
Conducting Price Studies
In today’s world, pricing is a multidimensional phenomenon that has to take into account benefits and additional benefits compared to therapeutic alternatives, customer preferences and reimbursement regulations. We conduct pricing studies for you in the top 5 EU countries taking into account customer preferences, the prices of comparator therapies as well as payer preferences.
Benefit Assessment
Early benefit assessment according to AMNOG pursuant to § 35a SGB V was introduced in 2011 and is the most important instrument for the assessment and negotiation of reimbursement amounts. Over time, requirements have become more and more complex and elaborate. We will safely guide you through the jungle of clauses and methodological requirements laid down by the legislator, the Federal Joint Committee and the Institute for Quality and Efficiency in Health Care (IQWiG). Based on our many years of expertise in this field, we advise you on the specifics regarding orphan drugs, ATMPs, hospital medicinal products and reserve antibiotics. We support you throughout the AMNOG benefit assessment process and prepare benefit dossiers for you that present the value of your medicinal products in the best possible way, thus creating the basis for the highest possible reimbursement amount.
Discount Agreements
Discount agreements and tenders for discount agreements are a central means of cost reduction and market access in Germany, especially for generics. Participation in the tenders of the health insurance funds is indispensable for many active substances in order to be successful in the market for generics. We provide you with comprehensive advice on the tenders and analyse the chances of success for you with regard to markets and active ingredients that are of interest to you.
Hospital Reimbursement– New Examination and Treatment Methods (NUBs)
Reimbursement of medicinal products and medical devices in inpatient care is subject to an assessment in terms of health economics for medicinal products as well as an additional benefit assessment for medical devices of risk classes 2b or 3. In terms of health economics, new examination and treatment methods (NUBs) are examined to determine whether they are already covered by the applicable case-based flat rates of the DRG system (diagnosis related groups). In case of NUBs, additional fees are paid outside the hospital budget, unless they are already covered by the DRG system. NUB applications for separate reimbursement may be submitted by hospitals to the Institute for the Hospital Remuneration System (InEK) until October 1st of each year. Applications during the year may only be submitted for gene therapies (ATMP).
In addition to the cost assessment, a benefit assessment is carried out for medical devices in accordance with § 137h SGB V, if NUB applications have been submitted for a new medical device of risk classes 2b or 3. We offer you all relevant services relating to the reimbursement of medicinal products or medical devices in inpatient care. This includes advice on NUB applications as well as preparation for the benefit assessment.
GKV-FinStG cabinet decision: Power shift in favor of the statutory health insurance companies expected
The German cabinet, the coalition group of ministers, has decided
