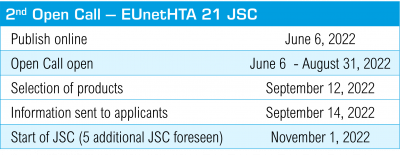
EUnetHTA21 offers early dialogue Joint Scientific Consultations (JSC) in parallel to the EMA scientific advice for medicinal products which are to undergo the EU Joint Clinical Assessments starting in 2025. In 2022, five JCS slots are available. Health technology developers can apply for a JCS during the open call from June 6 to August 31, 2022. Products are to be selected in September 2022 and JCS are scheduled to start in November 2022. Click for more information>>>