Since the beginning of 2025, member states of the European Economic Area (EEA) have been working together on joint clinical assessments (JCA) of new medicines. Recently, the first 9 JCAs were announced, assessing 2 ATMPs with Orphan drug status and 7 oncologics.
Germany, France, and Ireland are currently among the most active HTA authorities. Germany’s Institute for Quality and Efficiency in Health Care (IQWiG) is involved in 4 oncology assessments: as Assessor in 3 and as Co-Assessor in 1. The first report has been finalized already and is expected to be published in Q2 2026. Later this year, the Annual Work Programme for 2026 is expected, in which the planned number of Joint Scientific Consultations (JSC) and JCAs and the request periods for JSCs will be published.
Once finalized, JCAs will be used by all EEA states, which includes all EU member states and Norway, Iceland and Liechtenstein, as basis for national benefit assessments – a major shift in how evidence is evaluated across Europe.
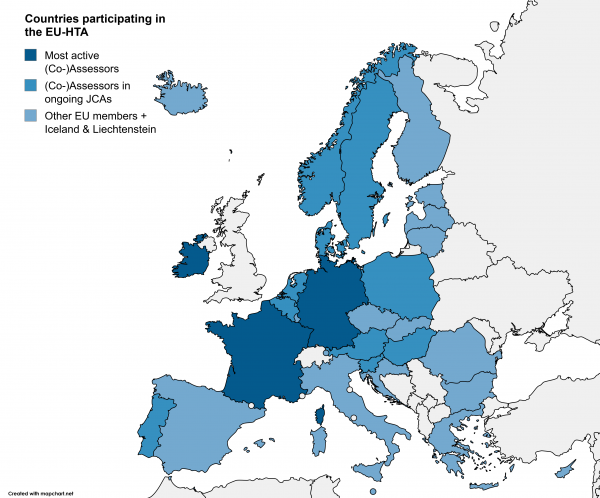
Figure 1: Countries participating in the EU-HTA. Countries functioning as (Co-)assessors in currently ongoing HTA procedures are highlighted.
The HTA Coordination Group (HTACG) is the main administrative body that is responsible for implementing the EU regulation. It encompasses four subgroups that focus on JCAs, JSCs, the identification of emerging health technologies and methodology, respectively. Each group is composed of member states’ representatives. These groups are in close contact with the HTA Stakeholder Network, which includes patient associations, non-governmental organizations and health technology developers as well as health professionals. Administrative, technical and IT support for pharmaceutical companies and other stakeholders is provided by the secretariat. Together, these groups and networks form the backbone of EU-HTA’s administrative and methodological framework.
To further support the implementation of EU-HTA, the HTACG has published a webinar explaining the JCA procedure in detail as well as guidance on using the HTA IT platform. The Webinar also includes updates and learnings from the currently ongoing procedures and features sections answering questions from the participants This is a helpful resource for all stakeholders preparing for the new European HTA framework. All information published by the HTACG can be found here:
This is a first glimpse of how EU-HTA collaboration is starting to take shape. These first joint assessments signal the beginning of a more coordinated approach to evaluating new medicines across Europe. By sharing expertise and aligning methodologies, EU member states and EEA countries aim to make health technology assessments more consistent, transparent, and efficient — setting the stage for future collaboration and innovation. The new procedure holds a lot of potential but also poses challenges and difficulties. Deadlines for pharmaceutical companies are tight, and requirements are complex. Good preparation and expert advice are key for successful execution.